Excess Reactant
After all you are just one step away. Checkout JEE MAINS 2022 Question Paper Analysis.
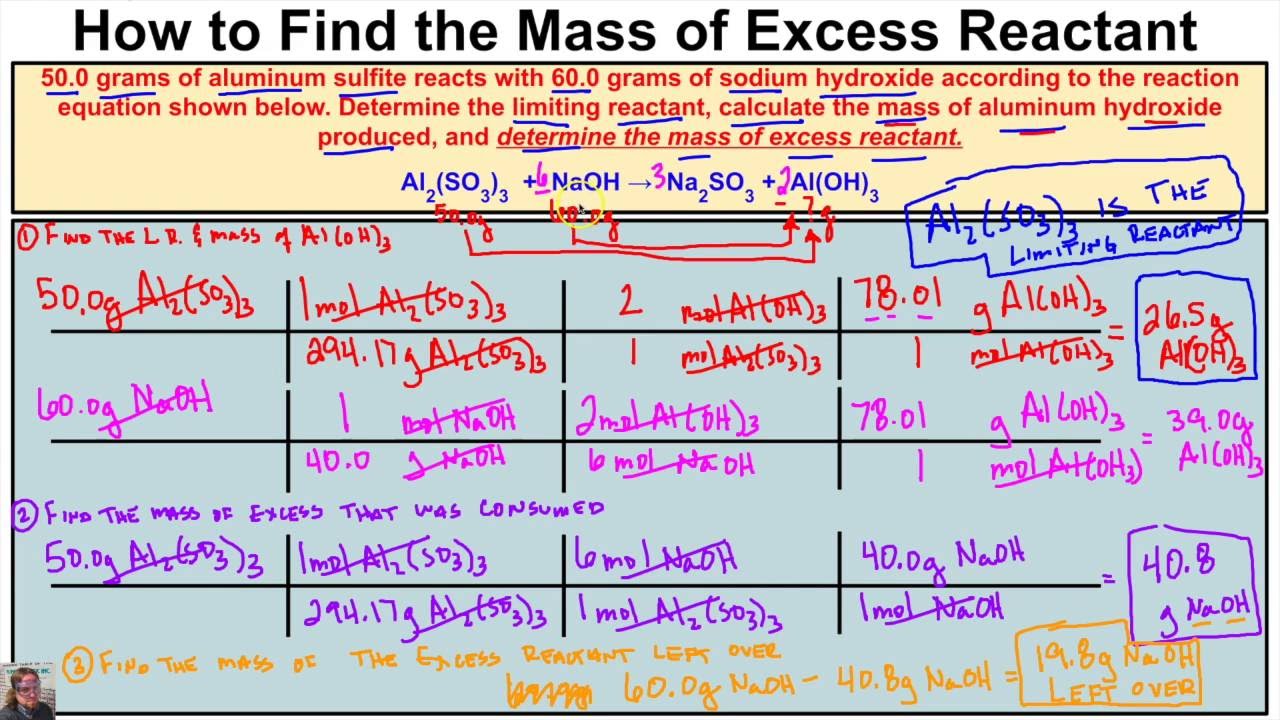
How To Calculate The Mass Of Excess Reactant Left Over In A Chemical Rea Chemistry Class Chemical Reactions Ap Chem
The reagent that is completely used up or reacted is called.

. It is also known as an excess reagent. Identification of the limiting reactant makes. This chemistry video tutorial shows you how to identify the limiting reagent and excess reactant.
- What is an excess reactantSometimes you may encounter limiting reag. This is called the excess reagent. 05 moles of aluminum are reacted with 09.
This is financially efficient when one of the reactants is very cheap. Now the mass of the excess reactant that is consumed in the reaction and the mass that is unreacted should be. The surplus reactants are referred to as excess reactants since they are in excess of what was necessary.
Reactions are often carried out in such a way that one or more of. Then subtracting the amount of moles you actually got for the excess reactant minus the amount you got using stoichiometry to convert the moles of the limiting reactant to. Now you need to wfor recognizing that what was the reactant that was fully consumed when reaction.
Excess reactants A good way to ensure that one reactant fully reacts is to use an excess of the other reactant. Known Amount of Limiting Reactant. - What is a limiting reactant.
It shows you how to perform stoichiometric calculations an. As a result an excess reactant can be defined as a chemical that is not. Limiting reactant problems So in the above problem O2 is the limiting reactant because limiting reactant reactant that produces least ml of product.
2 x 30070 g C2H6 7 x 31998 g O2 Calculated Amount of Excess Reactant. Find the limiting reactant when. In this video we are going to answer these questions.
An excess reactant is a substance that is left over after the reaction of a chemical equation has taken place. An excess reactant is a substance that is not wholly consumed or entirely reacted in a chemical reaction. Identification of Limiting And Excess Reactant.
You need to start with the limiting reactant and. In conclusion the SiO 2 it is the limiting reagent since when all the excess C is consumed more SiC would be generated. The limiting reactant or limiting reagent is a reactant in a chemical reaction that determines the amount of product that is formed.
An excess reagent is one that is present in large enough quantities that it does not restrict the outcome of the reaction. In a chemical reaction reactants that are not used up when the reaction is finished are called excess reagents. The reactant forming larger amount of product is the excess reagent.
An example of this would be when you mix a cup of salt and sugar to create a rock. 107 g C2H6 Leftover Excess. So far we have assumed that a given reactant is completely used up during the reaction.
Answer link Fir3hawk Oct 26 2016 The reactant that. The other reagent which is completely used up is called the limiting reagent. The amount of product formed is.
397 g O2 Mass Ratio. The particular challenge of this classic process intensification case lies on that reactant is lighter than product and is easier to be taken away from the reaction in such a way the conversion of. This chemistry video tutorial explains how to find the amount of excess reactant that is left over after the reaction is complete.

Introduction To Limiting Reactant And Excess Reactant Science Sciencewithtylerdewitt Tylerdewitt Tu Chemistry Help Apologia Chemistry High School Chemistry

Stoichiometry Limiting Reactant Excess Reactant Stoichiometry Moles Module 6 Learning Psychology Science Classroom School Work

Theoretical Actual Percent Yield Error Limiting Reagent And Excess Reactant That Remains Youtube Ch 8 Teaching Chemistry Chemistry Notes Chemistry

Excess Reactant Easy Science Chemical Reactions Easy Science Excess
No comments for "Excess Reactant"
Post a Comment